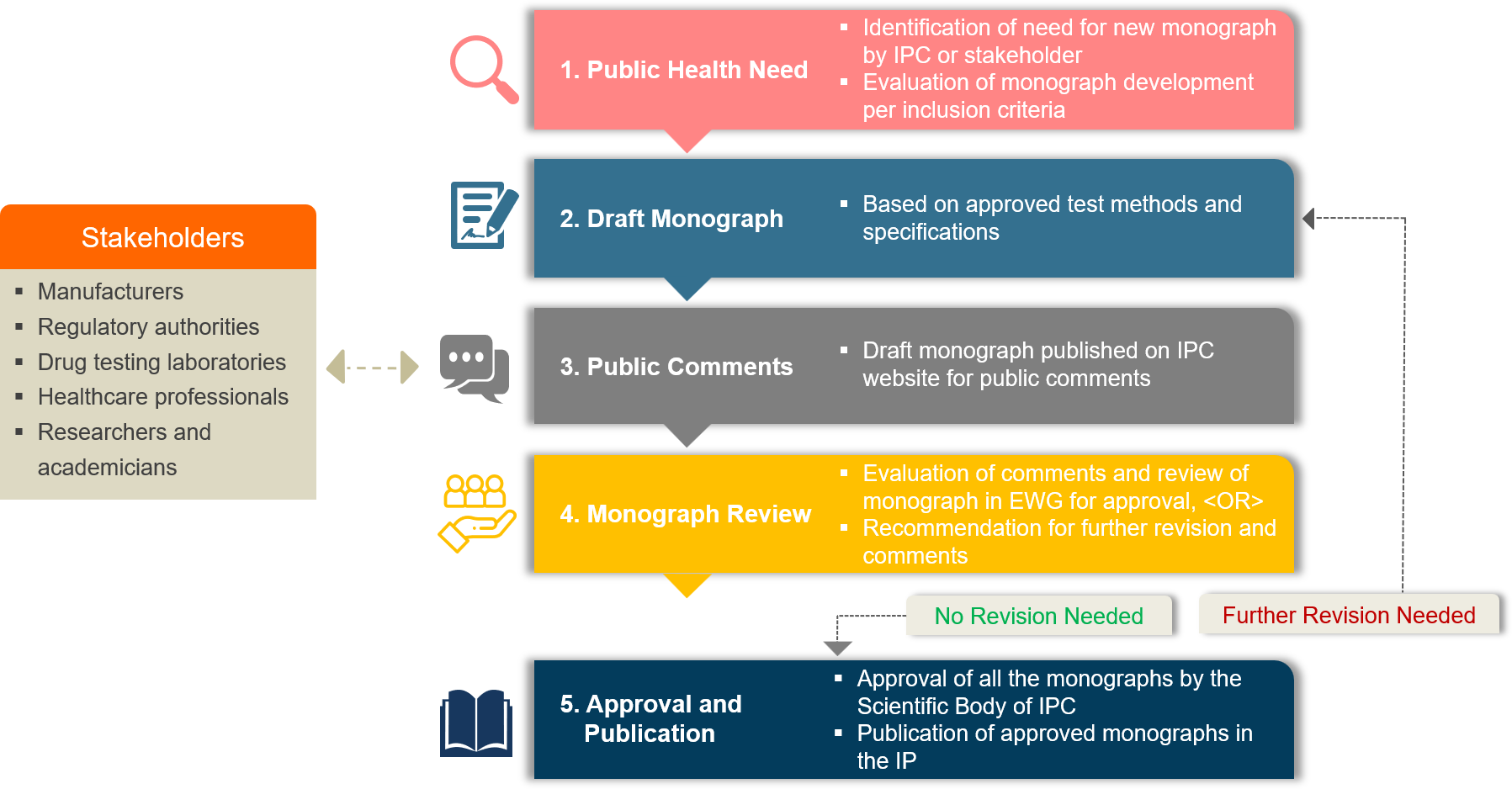
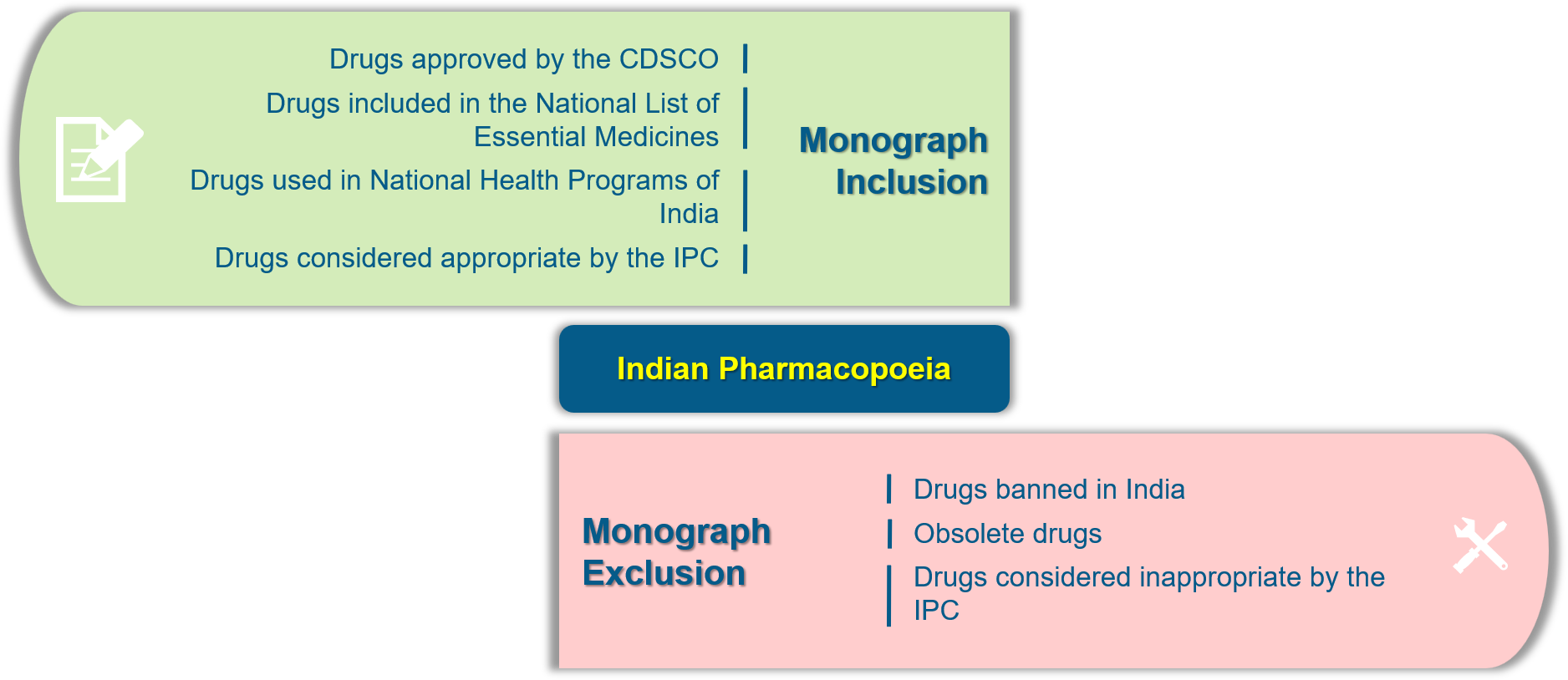
Monograph Development Process
The development of monographs commences by drafting them based on validated analytical procedures and specifications approved by the regulatory authority and donated by the manufacturers to IPC. The drafted monographs undergo review by specialized Expert Working Groups (EWGs) having members from regulatory authorities, pharmaceutical industry, drug control laboratories, health authorities, and research organizations. Members of the EWGs are required to uphold strict confidentiality regarding all the material, knowledge, information, and data generated and/or exchanged during monograph development process. Throughout the monograph development process, pharmaceutical manufacturers play a key role starting from sharing of approved drug specifications for the proposed monograph, providing test samples for method verification purposes, donating candidate material for reference standard development, and offering technical inputs on the draft monograph specifications before finalization.
To ensure transparency in the standards-setting process, proposals on new monographs and monograph revisions are publicized on the IPC website (https://ipc.gov.in) besides following the conventional approach of obtaining comments through consultations. The IPC and EWGs evaluate the comments received from the stakeholders on the draft monographs to assess their suitability and acceptance. Any necessary additional revisions are made by the IPC and updated monographs are once again made accessible online on the IPC website. If no further revisions are needed, the monograph is approved and prepared for publication in the IP. The IPC Secretariat evaluates whether the drug standards are appropriate for publishing in the IP after consulting with various EWGs and the IPC Scientific Body. Monographs are published in the IP upon approval by the IPC and are considered official from the date mentioned in the IP.